Progress your programme with our unique combination of inhaled development expertise, formulation science and device technology.
We offer a fully-integrated approach to support you in the development of your inhaled medicine.
Services
Formulation
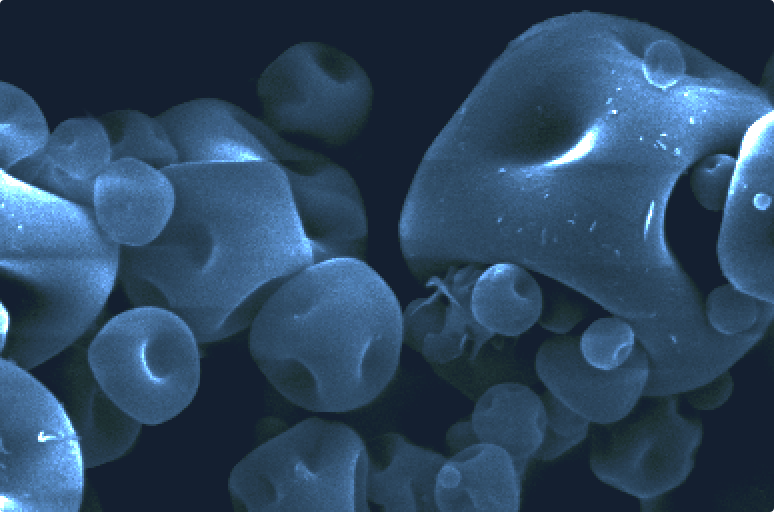
Inhaled formulation development is complex and challenging. We can help you overcome these challenges with our formulation technologies and deep scientific know-how to maximise the probability of success for your product.
We offer formulation development for DPI, pMDI and nebuliser products for small molecules, biologics, complex combinations and generic products.

Pharmaceutical Analysis

Our large, experienced inhalation group of analytical scientists can undertake the comprehensive array of test methodologies and physical properties characterisation required to support the development of your inhaled product.

Device Platforms

The optimal choice of drug delivery platform technology is critical to the success of an inhaled development programme. Making the right choices at each development stage can have a significant impact on the development timeline, cost and probability of success for the programme.
We have developed commercially-available DPIs, pMDIs and nebuliser devices to deliver a broad range of complex inhaled therapies. See how our device platform technology can support your inhaled programme.

Process Development & Technical Transfer

We can support your programme by developing robust manufacturing processes, scaling-up from laboratory to pilot to commercially-representative scale, ultimately enabling seamless transfer to commercial manufacturing organisations.

Product Manufacturing

We can manufacture your drug product and medical devices for development studies and clinical trials using our in-house GMP facilities. For late-phase and commercial products, we have a track record of managing manufacture and supply at 3rd party CMOs.

Regulatory Services

Our formulation, device and development services are supported by our regulatory, device vigilance and pharmacovigilance teams to safeguard your programme and ensure the smoothest path to product approval.


